In 2022 the Supreme Court corrected a grave error and overturned Roe v. Wade. Many states quickly enacted laws to protect women and unborn children from abortion or allowed preexisting pro-life laws to take effect. Other states, in contrast, doubled down on abortion extremism by creating abortion “sanctuaries” and promoting abortion on-demand—paid for by taxpayers—through all nine months of pregnancy.
The data is clear: Pro-life policies save lives of both mothers and babies. But it is also increasingly clear that abortion drugs pose the single greatest threat to pro-life progress. The U.S. Food and Drug Administration’s (FDA’s) reckless removal of safety protocols from the “mifepristone + misoprostol” abortion drug regimen is the latest move in a long line of decisions that has created the current landscape. Without decisive action to protect women and girls from dangerous abortion drugs, lifesaving laws in pro-life states will continue to be undermined, unborn babies will continue to be killed, and women and girls will continue to suffer.
Current Landscape
The Centers for Disease Control and Prevention’s (CDC’s) latest annual abortion report shows that over 57 percent of abortions in the United States are done using abortion pills.REF The real number is likely higher because states with lax abortion laws (California, Maryland, New Hampshire, and New Jersey) do not report abortion data to the CDC. Online abortion businesses and online pharmacies ship pills across state lines and may not account for in-state health department reports.REF And unscrupulous actors freely promote and distribute abortion pills from illegal international sources.REF
The typical abortion-pill regimen is a two-part process. The first pill, mifepristone (also known as RU–486 or its brand name, Mifeprex) kills the unborn child by cutting off progesterone, which is a hormone required to support pregnancy. The second pill, misoprostol (brand name Cytotec), causes contractions to empty the uterus. The regimen is currently approved to be used for up to 70 days (10 weeks) into pregnancy. But online pill providers freely advertise these drugs for abortions beyond 10 weeks,REF despite increased health risks.
Politicized Approval Process
The FDA approved mifepristone in 2000. The process was controversial and politicized from the start. As detailed in a Heritage Foundation Backgrounder, it would be years before documents revealed the extent of the Clinton Administration’s highly unusual behind-the-scenes maneuvers to broker a deal between a U.S.-based drug sponsor and the European-based company that held the license for mifepristone and shepherd the drug through the FDA approval process.REF
The FDA “broke with precedent by not publishing the names of the experts who reviewed [mifepristone] for the agency,” and “did not publish the name or location of the company that will manufacture the drug.”REF A Chinese pharmaceutical company later claimed credit for producing the drug for the U.S. market.REF
The approval was done under a special accelerated process called Subpart H. Among other things, this meant that the drug was subject to postmarketing safety restrictions. But Subpart H approval requires a drug to address a “serious or life-threatening illness,”REF of which pregnancy is neither. Population Council, the mifepristone sponsor, objected to approval under Subpart H for this reason.REF Abortion advocates wrongly claim that chemical abortion is necessary to deal with pregnancy complications, but mifepristone was not approved to address pregnancy complications: In fact, it is contraindicated according to the FDA label.
The FDA-approved abortion-pill regimen includes both mifepristone and another drug, misoprostol, which causes contractions to expel the dead child. This promotion of the off-label, unapproved use of misoprostol (which is labeled to prevent stomach ulcers) is a departure from the FDA’s typical role in ensuring a drug is used safely for its intended purpose.
In 2007 the FDA established what is known as a risk evaluation and mitigation strategy (REMS) for certain drugs. REMS automatically applied to drugs—including mifepristone—that require safety restrictions.REF As of February 2025, 71 drugs are subject to REMS.REF
REMS Changes
The REMS have changed over the years. The biggest changes happened in 2016 and 2023.
2000 Approval. Mifepristone could be used up to 49 days (seven weeks) gestation, but only if dispensed by a qualified prescriber approved by the abortion pill manufacturer, Danco. This opt-in process essentially segregated abortion-pill distribution to abortion providers, not primary care doctors and obstetricians/gynecologists. Physicians had to be able to date the pregnancy and diagnose ectopic pregnancy, which requires an ultrasound. They also had to provide or arrange for surgical intervention in the case of complications or a failed abortion. Physicians could only dispense the pills in-person in a health care setting and have in-person follow-up with the woman. Physicians also had to notify Danco of serious adverse events (such as hospitalization or transfusion). REF
2016 Update. The Obama Administration loosened the safeguards. It did away with the requirement for in-person follow-up care to ensure the abortion was complete and a woman was not having complications. It approved non-physicians to prescribe mifepristone and did not require that the pill be ingested in the presence of a doctor. The FDA extended the limit from 49 days to 70 days gestation (10 weeks) and weakened reporting requirements so that only deaths—not serious adverse events—had to be reported.REF
2023 Update. The Biden Administration formally updated the REMS to align with what had been informal practice since 2021 under the guise of COVID-19 containment.REF There is no longer an in-person dispensing requirement, and pills can be dispensed in retail pharmacies, not just health care settings. The FDA formally sanctioned dangerous policies like abortion-pill-by-mail and telemedicine abortion without ever having to be examined by a doctor.REF
A Disturbing Data Story
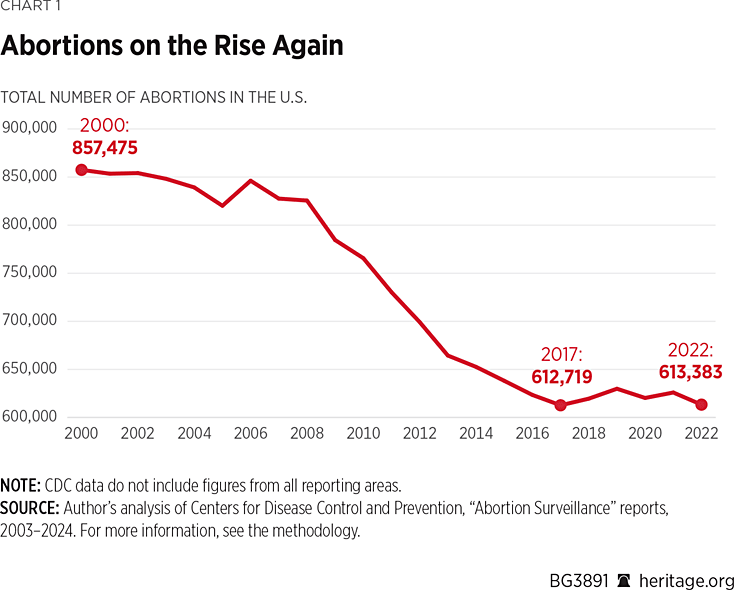
REMS changes are more than policy choices. They align with the explosion in the share of abortions that are pill-induced. And they align with slowdown in what had been an enduring decline in the total number of annual abortions during the past two decades. According to data from the CDC, the total number of abortions declined in the past two decades: 857,475 in 2000REF compared to 613,383 in 2022 (the most recent year of the annual abortion surveillance report).REF
But after hitting a low of 612,719 in 2017, the total number of abortions started trending upwards again. Chemical abortion and FDA policy choices are largely to blame.
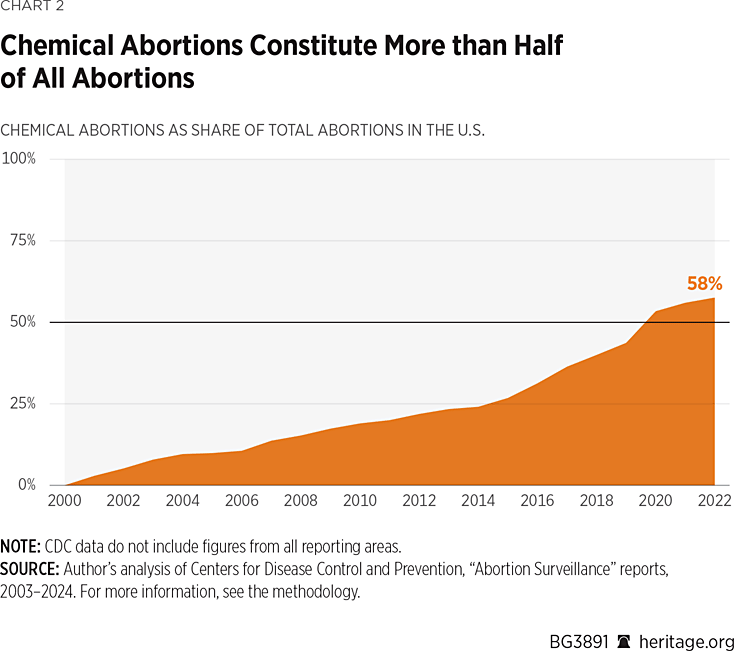
In 2001, chemical abortions made up a mere 3 percent of all abortions: By 2022 that number skyrocketed to 57.6 percent. From 2001 to 2016, the percentage of chemical abortions steadily increased. In 2016—when abortion-pill REMS were significantly weakened—the percentage climbed much more quickly.
The most recent CDC data is for 2022. This means data reflect the initial months of the post-Roe landscape in which several states moved to fully protect life. One study found that 32,000 lives have been saved so far since the Dobbs decision.REF But pro-abortion state policymakers and the abortion industry rely on interstate trafficking of abortion pills to undermine laws in pro-life states.
The data is clear. CDC abortion data, when viewed in the context of weakened safety protections, shows an association between an increase in the prevalence of chemical abortion and a reversal of the enduring decline in total annual abortions in the United States. This is a glaring warning sign to pro-life policymakers: If they want to protect women and unborn children from abortion, they must address mifepristone and the policies regulating its use.
FDA Turns a Blind Eye to Abortion-Drug Health Risks
Behind the data are real people: unborn children whose lives were cut short, and women and girls who often experienced serious side effects (or worse).
According to the mifepristone label, 85 percent of those who go through the abortion-pill regimen will have at least one adverse reaction such as nausea, fever, or vomiting (and often, multiple reactions). Eight percent of women may bleed for more than 30 days.REF
Abortion drugs are not safe. The complication rate from abortion pills is four times that of a first-trimester surgical abortion.REF Serious complications include severe bleeding, infection, and undiagnosed ectopic pregnancy.REF One study found that between 2002 and 2015, emergency room visits following a chemical abortion ballooned by more than 500 percent.REF Mifepristone is associated with 36 deaths,REF thousands of serious adverse events, and more than 500 known life-threatening complications,REF although some variance in this number is certain due to weak state and federal reporting requirements. According to mifepristone’s own FDA label, roughly one out of every 22 women who take the drug will end up in the emergency room.REF
The FDA has chosen to make mifepristone more widely available despite the significant health risks it poses to women and girls. The FDA’s reckless embrace of do-it-yourself mail-order abortion makes this dangerous drug even more risky.
Inaccurate Pregnancy Dating. Online prescribers rely on women self-reporting their estimated gestation. Without an ultrasound, the prescriber cannot accurately date a pregnancy.REF An estimated due date is established by first determining the first day of a woman’s last menstrual period (LMP) and verifying by ultrasound. At an online pharmacy, this is done by self-reporting LMP with just a few clicks of the mouse—no doctor’s visit and no ultrasound. The American College of Obstetricians and Gynecologists (ACOG) notes that approximately one-half of women incorrectly recall their LMPs, and in one study 40 percent of women had their due date adjusted following an ultrasound due to a discrepancy of five or more days of LMP dating.REF Online pharmacies are undoubtedly shipping abortion pills to women who report being within the approved 10-week gestational time frame but are actually beyond the cutoff.
Blood-Type Compatibility and Future Fertility. Determining blood-type compatibility is an important piece of standard prenatal care early in pregnancy. If a mother is Rh-negative and her baby is Rh-positive, a woman’s body will make antibodies to destroy the Rh-positive blood. During a first pregnancy there typically is not enough time for enough antibodies to develop and cause problems. But in subsequent pregnancies, more antibodies can be made and put an unborn baby at risk of serious health problems and death. A RhoGAM shot during each pregnancy and after delivery is needed to prevent a woman’s body from initiating the antibody response.REF
ACOG recommends RhoGAM if indicated, even if a woman ultimately has an abortion, miscarriage, or ectopic pregnancy.REF The National Abortion Federation recommended RhoGAM for abortions occurring after eight weeks until 2022, at which point it raised its recommendation to 12 weeks.REF Both organizations support telemedicine/mail-order abortions despite the inability to determine blood-type compatibility under these circumstances, ultimately putting women’s future fertility and the health of their subsequent children at risk.
Coercion and Abuse. Weakened safety protocols for abortion drugs makes it easier for pills to fall into the hands of bad actors, opening the door to coerced and forced abortions. During a telemedicine appointment, a provider has no idea who might be just out of view of the screen. A private in-person visit with a doctor might be a woman’s only chance to frankly discuss her uncertainty or reveal any pressure she might be receiving from a coercive partner, boss, or parent. Online pharmacies that will mail abortion drugs to anyone with just a few clicks of a mouse have no way to verify if the woman requesting pills is truly eligible—or is a pregnant woman at all. Coerced and forced drug-induced abortions are not theoretical. The Heritage Foundation has compiled a list—which unfortunately continues to grow—highlighting publicly documented cases, including those in which men attempt to induce a chemical abortion without a woman’s knowledge.REF
Recent Deaths Reveal Real-World Consequences
The FDA repeatedly weakening safety protocols has real-world consequences.REF
In 2022 Amber Thurman, a pregnant mother in Georgia, obtained abortion pills from a clinic in North Carolina. Her body did not expel all of her child’s tissue during the abortion— a well-known known complication—and she developed an infection. She did not receive a procedure to remove the rest of the retained tissue in time and died. Abortion advocates have tried to blame Georgia’s pro-life law for Ms. Thurman’s death, claiming that doctors were unable to intervene. This is, of course, not true: No state, including those with strong pro-life laws, prohibits doctors from treating life-threatening medical emergencies, miscarriages, or removing retained tissue.REF
The same year, another Georgia woman, Candi Miller, also died after taking abortion pills. She ordered them online from an overseas supplier and was never evaluated in-person by a doctor and never had an ultrasound to verify how far along in pregnancy she was. Despite suffering at home for days after taking the pills, her family says that she did not seek medical care because she was afraid of facing legal consequences. She never should have been afraid of seeking help, because no state places criminal penalties on women seeking care from an abortion complication. Miller died at home, and while an autopsy could not reveal a specific cause of death, it found that she had retained tissue in her uterus. She also had painkillers, including fentanyl, in her system. Had she sought care in time, doctors might have been able to help her sooner.
Alyona Dixon, a woman in Nevada, also died after taking abortion pills. She developed sepsis following an abortion in 2022.REF Despite her symptoms, she was not given a pelvic exam or seen by an obstetrician/gynecologist when she initially sought treatment at an emergency room. By the time she sought treatment at a different hospital the following day, it was too late.
Deliberately Collecting Less Data
In the 2016 REMS change, the FDA said that only deaths associated with mifepristone—not deaths and serious adverse events—must be reported by prescribers. This made an already insufficient reporting system even weaker.
A woman experiencing an abortion drug complication is most likely to turn to an emergency room rather than the abortion-drug prescriber at an abortion clinic, pharmacy, or telemedicine website. While certified prescribers are required to report deaths to the FDA, emergency room practitioners are not (and might not even be aware that a reporting system exists in the first place). There is no way to know how often emergency rooms and other facilities fail to report complications.
An emergency room might not know that a woman is undergoing an elective abortion as opposed to a miscarriage. One study reviewing Medicaid data found that more than 60 percent of abortion-pill emergency room visits were miscoded as a miscarriage, and that these women were twice as likely to need surgical intervention and at greater risk of being admitted to the hospital due to complications.REF
Even when doctors want to voluntarily report adverse events associated with mifepristone to the FDA’s Adverse Event Reporting System (FAERS), the process is often too cumbersome or time-consuming, and doctors do not have enough information to complete forms because they were not the original prescriber.REF By its own admission, the FDA says that FAERS data “has limitations” and “cannot be used to calculate the incidence of an adverse event or medication error in the U.S. population.”REF Here, the FDA gets it right. FAERS data undeniably undercounts mifepristone complications.
One study of mifepristone adverse-event data obtained via the Freedom of Information Act (FOIA) found that of thousands of reported events, “the surgical management of over half the complications was performed by someone other than the abortion provider, yet treating physicians are not required to report complications. Few reports were generated by those in Emergency Departments and hospitals who treated complications.”REF In other words, even if the FDA returned to requiring prescribers to report adverse events and not just deaths, undercounting would still be a problem because the abortion provider often is not involved at all once the pill has been dispensed.
The math simply does not add up when taking other sources into account. Researchers compared Planned Parenthood reports of mifepristone complications to FDA adverse event reports. In a two-year period, Planned Parenthood reported 1,530 serious adverse events, and another 330 adverse events were identified through FOIA requests (from all providers, not just Planned Parenthood). During that same time, the FAERS system reported only 664.REF Yet the FDA later defended its decision relying on inadequate FAERS data when it moved to weaken the REMS in 2021, pointing to a “small” number of adverse events.REF
The circular reasoning at the heart of the FDA’s decision-making is unacceptable. Removing a requirement that most adverse events be reported will, naturally, lead to fewer such reports down the road. That does not mean that the events are not still occurring. And it leaves the FDA blind to the chance that weakened safety protocols actually leads to more adverse events. The FDA did not follow the science; it manipulated the process.
Time for the FDA to Revisit Mifepristone
Members of Congress have rightly proposed policies like the “Save Moms and Babies Act” to block the FDA from removing safety measures like the in-person abortion-pill dispensing requirement.REF There have also been efforts to accomplish the same goal through the appropriations process.REF Such legislative efforts would immediately save lives.
The ball is not only in Congress’s court. The FDA should at the very least restore the REMS safety protocols as they were under President Donald Trump’s first term given the increased harms of abortion pills to women and girls compared to surgical abortion. It should once again require that all serious adverse events—not just deaths—be reported to the FDA. This should be a high priority to protect the lives and health of women due to the explosion in chemical abortion incidence and opportunities for abuse.
Half of the states have made great progress in protecting life in post-Roe America.REF But the widespread use of abortion pills, freely flowing across state lines, limits the reach of otherwise protective pro-life laws. The ultimate victims of dangerous abortion drugs and the FDA’s continued manipulation of safety rules are women, girls, and unborn children. It is not too late for the FDA to change course to follow science—and the law.
Melanie Israel is a Visiting Fellow in the Richard and Helen DeVos Center for Life, Religion, and Family at The Heritage Foundation.



